About This Episode
How do you recreate a brain circuit in a dish, and what can it unlock about our minds? Neil deGrasse Tyson, Chuck Nice, and Gary O’Reilly explore the frontier of neuroscience with Stanford neuroscientist Sergiu Pașca, to break down stem cells, how the brain forms itself, and assembloids: self-organizing brain circuits.
What exactly is an organoid? We break down how these new, useful tools for research are made. How do you turn back time on a cell in order to create stem cells? Do cells have their own clock? Learn how brain cells organize themselves according to a blueprint and how cells in a dish know the human gestational period. How do these cells know where to go, how to organize, and when to evolve? Are we simply unlocking instructions already written inside them?
We explore how assembloids are helping scientists model disorders like forms of severe autism and schizophrenia, complex diseases that don’t have a non-human proxy. Can these models let us test medications? Can we observe pain, coordination, and even emotional processing in these neural mini-systems? Learn how Sergiu’s newest work studies a proxy for the sensory circuit and the nuances of how we feel pain. Is pain a feeling or an emotion?
Could we one day use a patient’s own cells to build a personalized avatar brain for drug testing? What happens when assembloids grow old—do they age like real brains? Do they feel pain? Could they eventually learn? As we probe deeper into the biology of the mind, bigger questions arise: What are the ethics? Learn how this technology could revolutionize how neuroscience is studied and what we have already uncovered.
Thanks to our Patrons Andy Fleishman, Khal Khumalo, Mauritz Cronje, Kyle Stone, Kathleen Fitzpatrick, Ridge Glenn, Josh Gumina, Mike Evans, Eddie Trapp, Aaron Turetsky, Kenneth TRan, Deeks, Patrick Weglinski, João Bruno Agria Russo, Lester Fernandez, Shani, Jorge Zok Yepiz, Devin Waldron, Eric D, Luke Landry, Chase Snow, Micheal Wall (Bean), Stefan, Tori Kishman, James Sellers, Alex Hayman, Kyle Gosser, Maria Balog, Vytautas Jasas, Cainã Kubiaki, Ryan Berube, James Randall, QuirkyCollisions, Bryan Staley, Jake, James Fuller, Will Behave, Gordon Pluemer, Bob Dietrich, Pizza Pockets, Nip34, Sh40l1nmunk Munken, Nick Hanna, Lyman Jordan, Robert Brashear, Lemon Life, Azeem Ahmed, John Barry, Tomas Gomez, and Joss in Cambodia for supporting us this week.
NOTE: StarTalk+ Patrons can listen to this entire episode commercial-free.
Transcript
DOWNLOAD SRTSo, Gary, you keep digging up these neuroscience topics.
Yeah.
They seem endless.
Because we do not know yet all that we need to know.
This is good.
I thought you were doing it because you were trying to give me a message.
Which was?
Something’s wrong with my brain.
That, too, coming up on StarTalk.
Welcome to StarTalk, your place in the universe where science and pop culture collide.
StarTalk begins right now.
This is StarTalk, Neil deGrasse Tyson, your personal astrophysicist.
And today, it’s going to be special edition, which means we got Gary O’Reilly.
Gary.
Hey, Neil.
All right, man.
Yeah.
Always good to have you.
Pleasure’s mine.
Very good.
And Chuckie Baby.
Hey, man.
What’s happening?
All right.
Not good to have me, I guess.
Okay.
Okay, good to have you.
Gary, always good to have you.
And Chuck.
And Chuck.
Yeah.
So, you got a word here, assembloids.
Yes.
That sounds like somebody just made that up.
Just assembled it.
Yeah.
Assembloids.
Well, this is a show on assembloids.
What can you tell us about it?
All right, not that long ago, we did a show on Synthetic Biological Intelligence, or, if you prefer, Organoid Intelligence.
Organoid, yes.
I remember.
Right.
Now…
And those are, if I remember, like 3D cultures to build brain-like structures for bio-computing.
Right.
So, that’s what that was being used for.
For our future overlords.
Right.
All right.
So, this was putting biology onto technology.
This is something different.
However, it’s based around the Organoid Intelligence.
But this now becomes Organoids assembling together.
Oh.
Now, that then…
Self-organizing Organoids.
All right.
So, our guest today…
All right.
So, we just…
Okay, we’re getting into it, baby.
And what could go wrong?
Let’s not do that question just yet.
I’ll say that for the end.
Thank you.
Okay, go.
So, our guest today had the great idea of trying to get these Organoids to work together and coined the phrase Assembloids.
So, Assembloids is down to our guest work.
Now, these Assembloids can help us uncover the biological mysteries of our own minds.
So, are we just clumps of cells in the big Petri dish we can call life?
Yes, speak for yourself.
Sometimes it can feel like that.
So, let’s find our guest.
Did you say in the Petri dish of life?
Yes.
That was beautiful.
You’re welcome.
I’m just reading it.
And so, let’s see what mysteries have been sold, what mysteries are still out there.
And our guest, Neil.
So, drop in on our guest.
Yes, please.
So, we have Sergiu Pașca.
Sergiu, welcome to StarTalk.
It was great to be here.
Thank you so much for having me.
Yeah.
So, you’re a neuroscientist on StarTalk Special Edition.
We love neuroscientists because there’s a serious future opening up right before our eyes.
Yes, it is.
In plain sight.
A fresh frontier.
Fresh frontier.
A stem cell biologist.
Stem cells have been pretty much in the news on and off in the past couple of decades.
A professor of psychiatry and behavioral sciences at Stanford.
When I think about this, however, I think of a psychiatrist or behavioral scientist, they’re just putting someone in a couch or observing their behavior.
This sounds way more invasive than what it is you’re doing.
It sounds very puppeteerish.
Exactly.
And you’ve also been Tedding, that’s good, so we can dig you up in the Ted archives, correct?
Yeah.
Excellent.
And in 2023, you made a Knight of the Order of Merit of Romania.
Ooh.
All right, so let’s get back to basics here and put us all on the same page with what an organoid is.
So, an organoid is a clump of cells that is cultured in a dish, in a three-dimensional structure, and the name actually, organoid, which is organ-like, is supposed to suggest that it resembles an organ, so it’s similar in some function.
Of course, it’s not a replica of an organ, but is supposed to model features of an organ.
So, it’s a scaffold of an organ.
I guess like parts of an organ or like parts of the function of an organ.
So, for instance, for the brain, it’s not really a brain in miniature.
It’s not the entire organ in miniature, but it would be like parts or aspects of the brain that are being modeled.
And by the way, asteroids, they show up as stars in a photo because they’re so tiny, but they’re not stars, so they’re asteroids.
Boy, a little star.
Yeah, yeah.
So it’s organ-like, I guess, the same way you like, star-like.
Oh, wait, so those are organoids?
Yes.
All right, and so now you organize them in some way, or do they self-organize?
You give them instructions that they follow?
Well, I guess all of this work, to be honest, started with the ability to actually even grow stem cells in a dish if you were to step back and think how this all came together.
Stem cells, as you know, generally are derived from an embryo, and that has been certainly very difficult to do studies.
But then about-
We’re certainly politically fraught with issues related to the ethics of using human embryos.
And that was a big issue until you guys figured out, or your people figured out, how to create stem cells without-
Out of regular cells?
Out of regular cells.
So this is-
Well, that happened 20 years ago, almost 20 years ago, 19 years ago, when a Japanese scientist, Shinya Yamanaka, made this breakthrough discovery, where he actually showed that you could actually turn any cell that we have in our body, that is already differentiated, so like back in time to look like those embryonic stem cells.
And so almost like a cellular alchemy, so to speak, right?
Because it was like we always thought that it’s a one-way street, development is a one-way street, you never so like go back.
Just to run the same page, stem cells, while it’s always in the news, just as a reminder to the non-biologist, it is a kind of cell that you, under the right conditions, can turn into any other cell of the human body.
Is that correct?
Exactly, yeah.
Nerve cells, muscle cells.
Yeah.
And that’s why they’re prevalent in the embryo, because the embryo is manufacturing all the cells.
Right, okay.
Got you.
Stem cells have two properties.
They can turn into any other cells, and they can renew themselves.
So they can stay as stem cells for a very long time.
And of course, there are multiple levels of stem cells.
The first ones are the ones that are the most powerful.
They can turn into everything.
And then as you progress in development, they become more and more restricted in what they can do.
But the ones that are really in the beginning are the ones that you would like to have, so that you can ultimately guide them to become different other cells and tissues in the body.
So you put them in a time machine.
Is that that box that’s sitting behind you?
You say that, but how is that possible?
How are you able to take a brain cell that you’ve cultured and dial it back to a stem cell and then bring it into whichever area you need to bring it to?
So it was really a brilliant idea.
The build on work that was done before.
And essentially the experiment was like very simply done.
He just looked at the main genes that are expressed in the stem cells and then he said, let’s see which ones are really important.
So he took them and he put them in a, actually in the skin cell, took a skin cell and starting putting various combinations of those genes that are very strongly present in those stem cells.
And through this combinatorial experiment, he found out for, that if you put at the same time, pretty much confuse the cell, so to speak, and the cell becomes reprogrammed.
That’s why we call it cell reprogramming, because the cell is really reprogrammed to that state.
And it turns out that they have all the properties of those embryonic stem cells, but you can make them from anybody in a non-invasive way.
And of course, you can store them, you can ship them to others.
And so that was really a breakthrough for the field, because that opened up the possibility for the first time that you could get stem cells from anybody, from any patient, and then start to study it in addition.
I was finishing my clinical training around that time, and really to a large extent dropped everything, because my expertise, I’m a physician by training, my expertise is actually autism spectrum disorders and neurodevelopmental conditions.
And I was incredibly frustrated by the lack of models to study this disease.
There are animal models, but what is an animal model of autism?
I mean, that has been a challenging aspect.
We can’t really access the human brain, right?
I mean, that is sort of like this curse, this unbearable inaccessibility of the human brain.
I mean, it’s behind the skull, and unlike any other organ, you can’t just go there, get a biopsy and study it.
So we were sort of like blocked, so to speak, locked into this state where we couldn’t really make progress.
And yeah, so about 16, 17 years ago, I came to Stanford mesmerized by the potential of this stem cells that we can make, which we called induced pluripotent stem cells.
And they started thinking, could we actually turn them into neurons from patients?
And then study whatever defects are characteristic of that disease, but outside of the human body.
And that’s really what enabled all of this.
So that blew open the whole field at that point.
Exactly.
They opened the whole field.
And in the beginning, just to make it clear, I got all the grants and all the fellowships rejected all the time, as this being absolutely insane.
How can you actually make neurons in a dish?
And then even expect to find something from a disease that is so mysterious.
Autism is a complex disease of social behavior.
What are you going to see actually in a dish?
So we’ll get back probably to this conversation, but it was actually key for us to focus on a disease where we actually knew what to expect, sort of like to calibrate.
And that sort of like started this entire journey.
And in the beginning, most of these experiments were very simple.
You would take the stem cells from patients that we derive in a dish, and then kind of like spike in various molecules in a dish.
So like guide them to try to become neurons.
And those differentiation experiments were easy.
But then, about 10 years ago, it became clear that we’re going to need more of the three-dimensional aspect of development to really capture even more complex features of the brain.
And that’s how some of these 3D cultures, which are now known as organoids, appear first.
So if the neurons are self-organizing, A.
How do they know that they’re self-organizing?
And how do they know where to go and be organized?
That’s a very good question.
Self-organization is a remarkable force of nature and biology.
And very often, when we do these experiments in a dish, to be honest, for a very long time, I was thinking like an engineer, in the sense that if you want to build something in a dish, let’s say a circuit, you better know the blueprint.
You better know the instructions and provide them at the right time.
And so you don’t start building a new house until you really have a very clear plan and the tools.
But what we realize with time is that in biology actually, you know, cells come with the instructions, you know.
So once you make a specific cell, cell actually comes with the instruction.
And then by connecting, let’s say, to another cell, it reveals another set of instructions.
And another one and another one.
And that’s why we call this process self-organization.
So which really is the formation of order structured from, you know, relatively homogenous elements, which, by the way, like talking of physics and chemistry, this was known from the 19th century.
I mean, there are classic experiments that show, you know, that molecules organize quite beautifully.
You know, the Riley-Bernard convection, I guess, is the classic example.
But biology just brings it to the next level.
And now organizes cells pretty much on their own.
So what you’re doing is you’re bringing these together in this culture, this 3D culture, where the message and directions are already resonant inside of the cell.
So when you put them together or group them, they basically do what they were going to do anyway.
Exactly.
Okay.
With, we want detail, which is we have to make the parts right.
If you don’t have the right parts, then of course they won’t know what to do.
What to do.
Actually, what we spend a lot of time generally is making the parts.
Let’s think about the human brain.
I mean, the reason why the human brain is remarkable is because it has all these parts, which are very different.
You know, unlike, let’s say, the liver.
The liver is relatively homogenous, right?
A few cell types, kind of like any part is like any other.
You look at the brain, and now you have thousands of cell types.
I mean, the recent estimates say that there are probably 2,000 cell types just in the human brain, right?
Scattered through all these nuclei and regions.
And the remarkable abilities of the brain really result from the cells interacting with each other.
So, in the early days, like 15 years ago, we were making just a few cells, like a few spinal cord neuron cells, or maybe a few cortical neurons.
But then we’ve never really leveraged the ability of the cells to connect with each other.
And so, that’s where, essentially, Assembloids came, where once we figure out how to make some of the cell types, some of these brain regions, putting them together essentially was unleashing new forces of self-organization, which is really what the brain does.
I mean, the brain builds itself at the end of the day.
You know, if you think about it, right?
And it reorganizes itself.
Like, if you damage a part of your brain, it will reorganize itself so that that function might be taken up someplace else.
At least early in development, yes.
Early in development, it will do so.
And then, the more you progress, the less you can do that.
The less that happens, right.
What if you leave your cultured brain cells in the dish for nine months, a year?
What happens to them then?
Do they just take care of business on their own, or do they just fade away?
Something crawls out of the Petri dish.
There you go.
You have the smartest dish in the world.
It will chase you down the corridor.
Get that fork away from me.
Well, that was something actually really fascinating that we discovered like almost 10 years ago.
So at one point, we were, you know, my lab was still like in the early days.
And at one point, we realized that, well, I mean, these are expensive experiments.
You have to keep feeding the cells, and I was running out of money in the lab.
And so I told everybody in the lab, I said, you better go in your incubators and like make sure that you’re not maintaining cultures that we don’t need.
We need to focus, we need to save money.
And then somebody in the lab comes and says, oh, should I also like remove the ones that are like 300 days old?
I was like, what do you mean like 300 days old?
It’s like, yeah, I mean, you know, I knew that we were keeping them for very long periods of time, but I had no idea that we could keep them for such a long period of time.
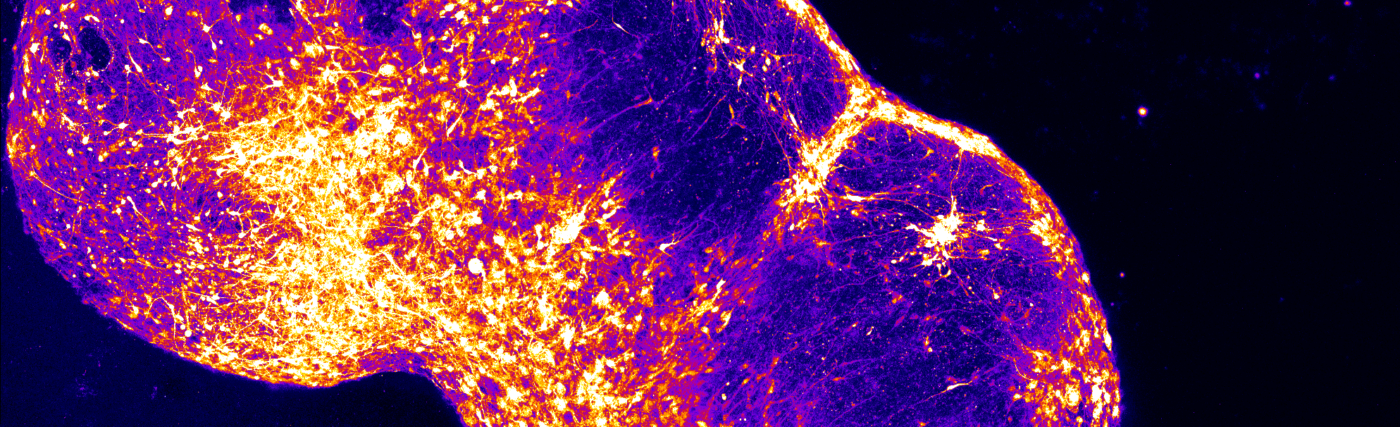
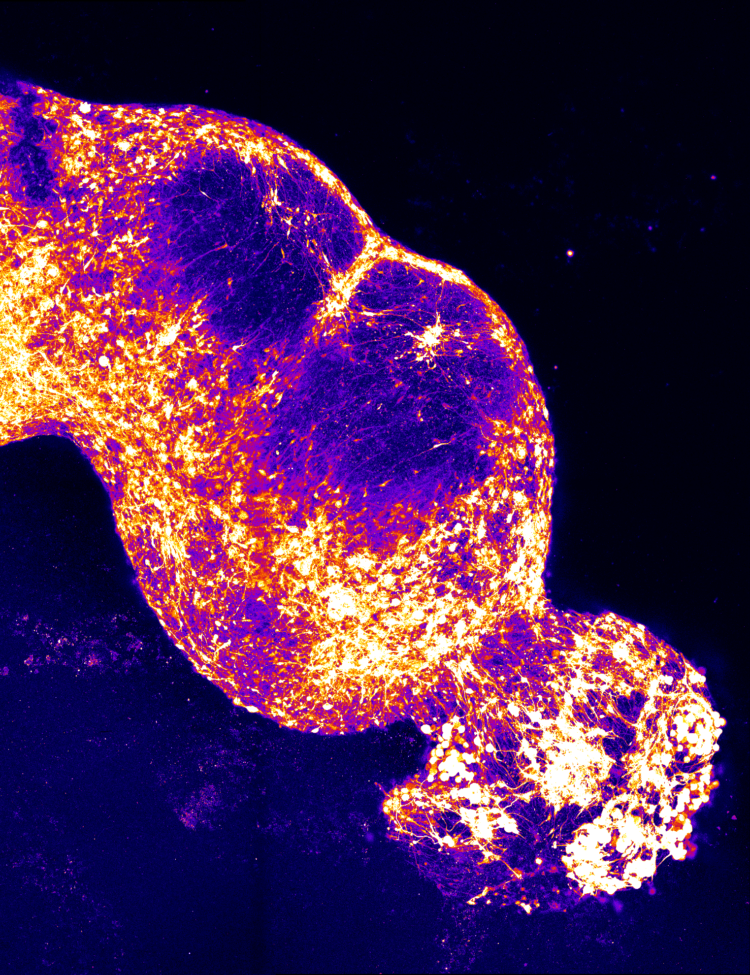
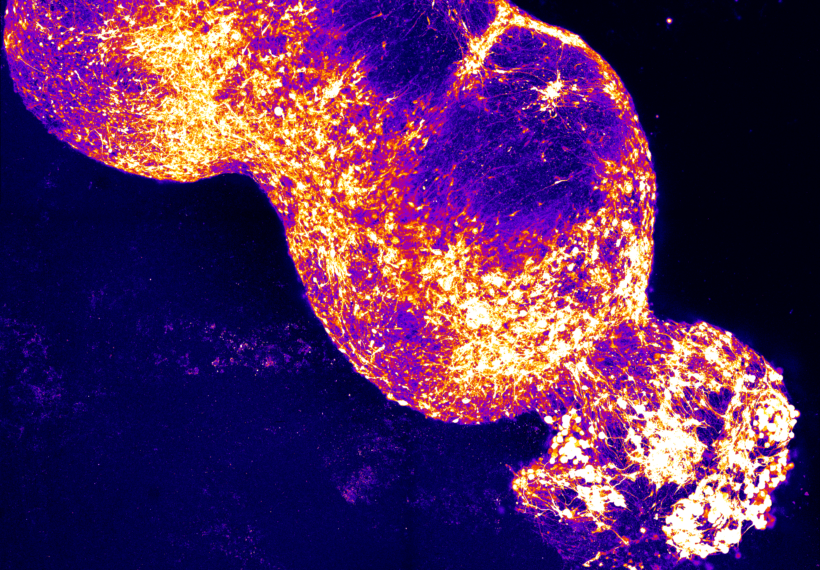
And it turns out that once you make this cluster of cells, and you know, it’s sort of like, I wish I could show you, I wish you were here in the lab and I could show you, or maybe I can try, but they look something like this.
All right.
I see it.
We see it.
They’re our lab.
They’re still like fixed, you see.
So they’re like relatively large clumps of cells.
They’re floating in the media, in the incubators.
You keep going and change media.
And then at one point we realized we can keep them for very long periods of time.
In fact, we maintain now the longest cultures that have ever been reported.
Like you can keep them for years.
And so now the question was, are they stuck in development?
Are they progressing in development?
And through a series of papers, we discovered something really fascinating.
It’s like they actually keep track of time really well.
So well that once they actually arrive at about nine months of keeping them in a dish, they actually transition in terms of their gene expression and some of the properties of the cells to a postnatal brain.
So it’s almost like they know that birth should happen.
It’s almost like we think that there is some sort of internal clock that keeps track of time.
Is this the brain clock that I’ve read about?
Yeah, this is the brain clock, exactly.
I’m fascinated now that these cells have the ability to understand basically a calendar.
I mean, because they’re not observing the sun going across the sky a day and a night.
Yeah, so what’s doing the taking inside the day?
So, I mean, you may think that this is surprising, but if you think about it, it’s not that surprising.
I mean, every time you make a human, you always make it in like 280 days.
And here’s the interesting thing.
If you take mouse stem cells, okay, or we have like chimp stem cells, and you differentiate them the same way in a dish, they’ll finish development in their own time.
But in that same time period reflects the gestation period of a chimp or a mouse?
Like it will be three weeks for the rat, and it will be like, you know, whatever is for…
So this is, I mean, evolution has actually selected, you know, very well, like this periods of development.
And so they’re intrinsic to the cells.
I think what we, what was surprising for us was that this happens also outside of the body, right?
Outside of the uterus.
Of course, this is not to say that all aspects of development are recapitulated.
I mean, there are all kinds of things that are coming, right?
What kind of information is that are coming, that are shaping development?
And we know that the more you invest in human brain development, the more the environment is important.
Like sensory information, right?
Like cognitive development, think about motor behavior afterwards.
But especially at early stages of development, everything is quite well regimented and goes according to a calendar.
Nobody knows what the clock is.
So nobody knows what the molecular mechanism of it is.
But it is somewhere in the cell.
It’s something that is counting somehow time.
And that’s why it’s such a great time to do neuroscience.
I’d like more people should like come and do that.
So where do these cells derive their energy from?
Because you talk about a clock, there’s not a battery in the back.
What is powering this?
Because they’re outside of the body.
They’ve not got the whole human system to back it up.
So we feed them a soup of chemicals that is made in the lab.
So we provide them glucose.
They need glucose and some of the amino acids.
We give them lipids, so they need fats.
So we just have, we call them cell culture media.
And how do you measure if and when they are expressing their prescribed function?
Because a neuron has a very specific function.
Absolutely.
How do you know that they are actually expressing that function?
So we do all kinds of things.
Like, first of all, we just look to see, very often, you know, cells, I mean, not very often.
All the time, cells have a signature.
You know, they express a certain combination of genes.
And so generally, the first question is, if you think you’ve made a cortical neuron, let’s say a neuron from the outer layer of the brain, how do you know that it’s a cortical neuron?
Well, first of all, you kind of like look at what genes it expresses and you compare it with what we know from a neuron in the actual brain.
Then you can look at how it looks.
There are often neurons in the cortex have sort of like a pyramidal shape.
We call them pyramidal neurons.
So you look, do they look pyramidal?
That means like a pyramid?
Yeah, exactly.
Pyramidenal, okay.
What do you call it for me?
Pyramidal, yeah.
They really look like a tiny pyramid.
An inverted pyramid, like that’s how they sit in the cortex.
So you look at this like the shape of the cell body.
Or the other thing is sometimes they move in very specific ways.
And that’s actually how the first Assembloids were actually looking at how cells are moving.
So here’s this interesting fact.
You know, you may think that, you know, you have all the cell types in the brain, right?
But they’re all made, you know, when you build the brain, they’re all made sort of like in their place and then they sit there.
Actually, it’s more, you know, a rule rather than an exception that cells do not reside in the place in which they’re born in the brain.
So there is a lot of movement.
So think about the cortex, okay?
Like the outer layer of the brain.
It has neurons that are exciting other neurons and it has neurons that are inhibiting other neurons.
And there is a very good balance between the two of them.
Too much excitation, you get epilepsy, right?
So, you know, think about that.
Now, here’s the interesting thing.
All the excitatory neurons are born there in the cortex, but all the neurons that are inhibitory, are built in a deep part of the brain.
And literally, during brain development, they start moving, crawling, for inches and for many, many months, until they arrive into the cortex, and then they kind of like establish that balance.
So, in order for you to build that cortex, it’s not enough just to make the excitatory cells, you have to make the inhibitory cells.
But then the question is, how do they come together?
How do they assemble?
Because that’s where the name Assembloid came.
And essentially, the vision was like almost, you know, 12 years ago, was let’s make these two parts of the brain, the one that makes the excitatory neurons and the one that makes the inhibitory neurons, and then just put them close to each other.
And hopefully, the cells will know what to do, because we certainly don’t know how to guide them to move.
And it turns out that exactly what they do, you put them together, and these GABAergic cells immediately start, like they have these processes, the cellular processes, they start to like smelling where the cortex is, and they literally start jumping.
You know, you see the cells, they literally spend three hours, they look in that direction, and then they make a jump, 40 microns, then they wait for another three hours, kind of like smell where the cortex is, make another jump.
And this process has never really been seen in humans.
This happens in the third trimester of life.
But this is what’s going on in every developing human being.
What you’re describing.
So Professor, what you’re saying is, basically we have a bunch of cells that are in a field, and they’re looking, and they recognize one another, and then they just start running to each other in slow motion.
Well, pretty much, pretty much.
Because they really come with instructions of how to do this.
And I think that’s what happens in development.
And that’s why you build a brain.
I mean, our brains may be a little bit different from each other.
But in the grand scheme of things, they’re quite the same, right?
I mean, we have the same structures.
It’s not like, you know, you have a thalamus in the spinal cord, right?
We all have pretty much in the same position.
So, in order for, you know, the brain to build itself that way, there are these remarkable forces that bring all the cells together over and over again every time you build a human brain.
Wow.
Okay, this is the last thing.
I’m sorry.
I know we got to move on to the next subject.
But here’s what is, like, percolating in my brain right now.
Is this, once you kind of perfect this technology, would you be able to then introduce these cells and have them go in and, let’s say, for instance, repair a part of my brain that kind of makes me so stupid I don’t believe in climate change or something?
Well, this sort of, like, self-assembly actually works really well early in development in the sense that all the cells are open to, like, connecting with the others.
But then it turns out that as you progress in development, the cells become less and less permissive.
We don’t have cells moving in our brain right now.
Okay.
You know, it’s just not very adaptive.
So the challenge is that if you start to add the cells into an adult, like, those circuits are already formed.
So it’s not that easy.
Gotcha.
To the dumbass circuitry.
The dumbass circuitry is fully formed and very, very strong, right?
However, if you’re able prenatal to identify brain disorders or any disorder in a child, you might be able during the gestation process to go in and make changes.
Exactly.
And that’s exactly what we’re doing.
Actually, even early after birth, because the human brain develops for, like, years, even after we’re born.
That’s amazing.
So if Professor…
That’s amazing.
All right, so Professor, you did some work and some research with cells, and you said you work with autism patients and the like, and there’s something called Timothy syndrome, which is autism and epilepsy, which seems like a terrible combination to afflict somebody.
It’s terrible for Timothy, damn.
But you then afflicted cells with this Timothy syndrome, is that correct?
Yeah.
And then reverse-engineered how you could find a way to work with and do basically what you said, take that away.
Right.
Is that, I mean, I am explaining that at all well, but you probably could do it better than I, if you would.
So, I mean, this goes back to, like, you know, the previous point when we were talking about how the stem cells were so, like, discovered and what their potential was.
So the question was, if you really want to model a disease, you know, you want to model a complex disease such as autism and epilepsy, you know, where do you actually start?
I mean, psychiatric disorders are mysterious disorder.
We still don’t know how, like, this, you know, starts and this complex social behavior arises in the brain.
So, actually, we thought we would start with genetics because one thing that we do know about many of these neurodevelopmental disorders is that they’re caused by mutations.
They’re caused by very severe mutations.
So there’s this rare, rare syndrome.
I mean, literally, there are about, we found about 30, 40 patients in the English-speaking world today, very few, but they have a mutation, this patient, in a protein that is actually a channel for calcium in the cells.
Every time a neuron communicates with another neuron, it opens up these channels, lets calcium in, and it essentially translates electrical information into chemical information inside the cell.
So it turns out that this patient have one single letter mutation in their entire genome, one single letter, that makes this channel open for a little bit longer.
That’s it.
It’s not all the time open, it’s not just slightly longer.
So the idea was that if you were to model this disease, you could make neurons from this patient, then look at them and actually monitor calcium inside the cells.
And if we were to see more calcium, it means that we started modeling the disease.
And that’s exactly what we did, because we wanted to really ascertain that we were really studying the disease process that is relevant.
So if you know the actual letter, and when you’re talking about that, you’re talking about the DNA sequencing.
So if you know the actual letter, why not do something like CRISPR, where you just go in and snip out the letter?
Well, sadly, you would have to change it everywhere in the brain.
Ah, there you go.
And that is not doable today.
And these patients are very severely affected.
I mean, they’ll have epilepsy, they’ll have autism spectrum disorder, they have a heart problem, so many of them would die because of a heart problem.
And so that’s where we started, with cells from these patients.
And then with these models that I’ve told you now, over the past 15 years, we kept building the models to be more complex and try to understand this disease.
And first we understood how calcium gets into the cells.
Then we saw that the cells are not moving right, they’re not connecting properly.
And about three years ago, which was one of the most interesting times in my academic life, was at one point we just accumulated enough information about the disease that essentially the therapeutic just became self-evident, so to speak.
Either just like look at it, and they would say, oh, this makes sense, this is what we need to do.
And so I don’t want to go into the details of how we think this, but it has to do with how this gene is processed inside the cells.
We’ve done a screen and essentially identify a tiny piece of a nucleic acid that if you add to cells goes right into them, changes the channel and essentially restores almost every single defect that we’ve discovered over the past 15 years.
Just like within a couple of days.
This is insane.
What you’re talking about.
He’s a Sherlock Holmes.
This is real detective work.
To work out that that is exactly what’s necessary.
You said it was obvious, but obviously it wasn’t, otherwise someone would have seen it a long time ago.
So if you were around in Frankenstein’s day, Frankenstein could just be the regular Joe on the street.
He would have walked out instead of like, he would have been like, hey, what’s going on?
How you guys feeling?
Are you mapping this with sort of an AI technology?
I mean, CRISPR is one tool, but there are others out there.
Is that what it is or is this just the empirical evidence from experiment?
It’s largely empirical.
I mean, we just accumulate enough information about the biology that at one point it became clear.
And it’s quite interesting if you think about it, because this could be the first psychiatric disease that has been exclusively understood with this human stem cell models, meaning by studying human brain cells outside of the body of those patients.
And so of course the question is like, how do you actually know that it would work?
Generally what we do is we use an animal model for the disease, right?
You have an animal model, you have a mouse that has the same mutation.
Well, it turns out that if you do this mutation in a mouse, it doesn’t really recapitulate the aspects of disease.
It doesn’t work that well.
So now what do you do?
You can also just go straight into a patient.
You want to make sure that it works in an in vivo setting.
And so that’s why one of the things that we’ve done over the past years is actually also develop transplantation methods.
Meaning that while the organets and the assemblies that we’ve been building are rather complex, they still don’t receive sensory input.
They don’t mature to the same level.
So what we started doing is actually transplanting them.
Meaning we essentially take the organ that we’ve made in a dish, but now we put it into the brain of a rat.
And then if you do it early in development, then the rat can actually grow to have about a third of a hemisphere to be made out of human cells.
You can literally see it on an MRI.
And you may think, oh, this is, you know, why would you even do that experiment?
Well, the reason is because now we actually have human tissue from patients in a living organism, and you can test the drug.
So what we did is we took the drug that we tested in vitro in a dish, but then injected it into the rat the way you would do it into a patient.
But then we looked at the effect on human cells, making sure that it doesn’t kill human cells or that it doesn’t do something else.
So that is like one way that allows us actually to test therapeutics in a way that is like safe essentially.
So the thing is, if you want to solve the issues of complex brain disorders, you’re going to need more complex assembloids.
Now, you’ve taken this assembloid up a notch, have you not, and daisy chained four organoids together, but then gone down the path of sensory.
If you could sort of expand on that for us, because I think this is absolutely fascinating.
You’re telling me they have feelings, is this what you’re telling me?
No.
Let the professor explain.
It turns out that if you think about brain disorders, some of them are sort of like hardware defects, right?
Parts are just missing.
Think about it in a stroke, right?
You lose parts of the cortex.
But most disorders that we consider today psychiatric, autism, schizophrenia, we think of them more as disorders of software, of communication between the cells.
So it becomes really clear that if you really want to capture those processes outside of the human body, we still like need to reconstruct those circuits outside.
And so this started like, you know, maybe five, six years ago when we thought could we actually build a circuit that is actually has an output, you know, really easy to measure.
So we decided to reconstruct the corticospinal pathway.
So that means, and you know this really well, everybody knows this, this is like biology textbook information.
You have a neuron in the cortex that generally goes all the way to the spinal cord, makes a connection or a synapse with the spinal cord neuron, and that spinal cord neuron goes to muscle.
You have essentially three neurons, two connections.
You stimulate the cortical neuron, information goes down to the spinal cord, to the muscle, the muscle contracts, right?
You know, it’s as easy as like text to biology.
So we thought, could we actually reconstruct this?
You may think that it’s easy, but here it is.
We don’t know how the cells find each other in development, by the way.
We have no ideas about the rules.
So what we did is we made an organoid that resembles the cortex, one that resembles the spinal cord, and then we made a ball of human muscle from a biopsy.
You can get a biopsy of muscle, build it as a ball, and then we put them all three together.
And it turns out that once you do this, those specialized neurons in the cortex, not every cortical neuron, but the ones that really go to the spinal cord, start to leave the cortex, find the motor neurons, then the motor neurons leave and find the muscle, and then the three preparation starts to contract.
Wow.
That was a three part assembly.
All right.
And that told us that even against the odds, because the probabilities for the cells to find each other is very, very low, and yet this works beautifully.
And you can actually stimulate the cortex and you get beautiful muscle contractions.
And we’ve been using this really in the last years to identify, for instance, how polio virus and other non-polio enteroviruses actually affect the spinal cord and cause paralysis, which is very difficult to study otherwise.
So it is a very important preparation.
You can add this polio virus and you can cause a paralysis of that circuit in a dish.
This work is not yet published, but it tells you just how useful a preparation like this can be.
It’s beyond useful.
I mean, what I’m trying to figure out, not figure out, envision is a time where we’ve mapped like everything, right?
So you have the layout.
Now, would there be a time because of what you’re saying that we’ll be able to go in, identify in a child that is developing in the womb, and then identify mutations, and then take the assembloids, put them into the child, and have those mutations corrected before the child is born?
Is that the deal?
Perhaps even an easier scenario for that.
Okay, go ahead.
Is that have a mutation, you know that a patient will have a serious mutation, you build an assembloid that models the disease of that patient without using the patient brain.
So like an avatar if you want, right?
I mean, that’s what an assembloid is if you think about it, right?
It’s an avatar for that circuit, simplified in a dish.
You test the drug or you screen for drugs.
Maybe you want to screen quickly for drugs.
And then you use that in a patient.
So now you can do that for every single patient.
You don’t have to actually do the process in any particular patient because now you developed a drug for the mutation itself.
Now just boom, boom, boom, every single person with that mutation gets that drug delivered and that’s how you…
Wow, that’s amazing.
But to get there, we do need to get a better understanding of how, because you see we’re quite…
Why do you have to understand why the cells do what they do?
They’re doing it.
You already know.
Why do you have to…
Are you just that newsy?
He’s a scientist.
That’s right, because he said, look at Neil, Neil’s looking at me like, how dare you?
He’s a scientist.
How dare you?
We don’t accept just what is.
I understand.
Go ahead, please.
If you actually think about like Richard Feynman, he famously said, and I’m sure you know this, that what I cannot create, I do not understand.
And you know, if you think a little bit about this, right?
If we cannot recreate the circuits outside, it’s got to be difficult for us to understand.
And if we don’t understand the biology, all the breakthroughs in medicine that came over the last decades, think about cancer in children, right?
In the 60s, 90% lethal.
Today, less than 10% lethal.
Why this entire revolution?
Molecular biology.
Because the tissue of interest was accessible.
You get the blood of these patients in leukemia or the tumor, you bring it to the lab and you deploy the power of molecular biology.
We in psychiatry and neurology are really the last ones because we cannot access the brain.
So my belief is that as we gain access to the brain, through this method and others, non-invasively, we’re going to be able to deploy the power of molecular biology and make breakthroughs in molecular psychiatry and neurology as we’ve done in cardiology and other branches of medicine.
That’s sort of like how I see it, but I may be wrong.
But haven’t you got an assembloid now that’s, like I said, a four-stage assembloid, but you’ve worked it so as it’s sensory and you can feel the understanding of pain and then how that becomes hypersensitivity or to the point where people do not feel pain at all.
Oh, okay.
I thought you meant like they’re going to have that little vial of assembloids just screaming in the middle of the night.
Why did you give me pain?
Not that one.
No, you’re right.
That one of the things that we’re trying, actually, this just came out.
I mean, we made the first assembloid in like 2017.
It took us three years to make go from two-part assembloids to three-part assembloids, the one with the motor that I was explaining.
And then it took us another five years to get to four-part assembloids.
Just because it’s technically more and more complicated.
And this is the pathway that sends us, you know, sends our information.
So think about it.
If you, you know, want to sense anything, even a painful stimulus on a finger, you have nerve terminals that are coming from neurons that sit close to the spinal cord.
They have receptors that sense that.
Then they send that information to the spinal cord.
The spinal cord shoots that information up to the thalamus in the middle of the brain, and the thalamus sends it to the cortex, and then you sense that something happened.
You know, that’s how it works.
So what we did is essentially we tried to reconstruct that from part.
So we made neurons that have some of these receptors, including receptors for pain.
So you know, the receptors for pain actually respond to capsaicin, you know, red hot chili pepper.
That’s why it’s like so hot.
So they have this specialized receptors, and you add capsaicin, and they just beautifully respond, like electrically.
But this had never been witnessed before, had it?
No, I mean, to put the entire circuit together has never really been done before.
What was it like for you to witness this the first ever time?
Well, the most beautiful part of it was, to be honest, once we made the parts, which took us years, you know, the four parts of the circuit, and then put them together, and it takes about a hundred days to make them, by the way.
And then another hundred days for the cells to connect with each other.
And then at one point, we started like looking at them and seeing like what’s going on.
And we’ve discovered something, you know, really remarkable.
The cells in the circuit become synchronized with each other.
So, initially, they were all sparkling, you know, in a non-coordinated way.
And then at one point, the activity just seems to be starting on one side and it goes, you know, one unidirectional.
So, the circuit is almost, you know, and there’s no stimulus, by the way.
You know, it’s almost like, which we know also from brain development, that the brain prepares itself before it even receives sensory inputs for what is about to come.
It’s almost like practicing.
So, it’s practicing to add, you know, the stimulus.
And then, the relevance for pain is that there are this interesting, maybe you’ve heard about this, neurologists discovered them, you know, in the past 20 years.
There are these patients that have mutations that make them either completely insensitive to pain, so they literally feel no pain.
And it’s really caused by a mutation in a channel, in a sodium channel.
Or they have the opposite.
They have this channel hypersensitive, so they’re hypersensitive to pain.
Both of them are obviously very bad.
So now what we did, we used CRISPR, because we were talking about CRISPR before, and genetically modified the cells in a dish to half the mutations that are present in patients.
Then put them together all four and started watching to see what happens.
And in the patients that have that hypersensitivity to pain, they’re very sensitive to pain, you just see the information going really, really fast.
The cells are super active and they sense it.
But in the ones that have no pain, it’s not like there’s no activity at all.
Actually what we found is there’s a lack of coordination.
The cells are essentially like lost that coordination.
So that’s why it’s so important to have the parts because really at the end of the day, the brain is more than the sum of its parts, obviously.
And so clearly in order to understand some of these disorders, we’re going to need to have some of these parts put together to get this emergent new properties.
So you’re not feeling pain.
That’s the Novakane movie, isn’t it?
Yeah, but we see this in certain people that, okay, I remember we did on the TV show and Neil has this crazy thing.
He could stick his hand in water, ice water.
I’m not, and I’m not saying it right.
Take a bunch of ice, add water to it, and it actually becomes colder than freezing, okay?
All right?
Then you put your hand in it and it burns your hand.
So we did an experiment and I stuck my hand in and he stuck his hand in and literally my hand started burning in what a normal person would have their hand burn.
Then he was able to leave his hand in there for a god awful amount of time, to the point where-
This is while you were squealing at the time.
Well, yes, I was because it burned.
It was not cool.
Not literally burned because it’s cold, not hot.
Right.
It’s not literally burned, but it felt like it was burning.
Okay.
But for you, for some reason, and you know, I just talked it up to him.
He got a lot more fat on his hands.
But seriously, it’s a matter of sensitivity to pain.
No.
Absolutely.
No, it’s not.
No.
What is it?
I didn’t say I didn’t feel the pain.
It’s just that I could deal with it.
Yeah.
Oh.
Well, that just changes everything.
Okay.
So explain to me the mind over matter aspect here.
Yeah.
And that’s a great point actually, because you see, this is not the only pathway for pain.
It turns out that we have at least two pathways in the brain.
One of them allows you to tell there is a painful stimulus.
You know, I sense it, it’s on my finger, or my hand is in the water, not my feet, right?
That’s the one that tells you that.
And then there is a second pathway that actually leverage other brain regions, the amygdala, the cingulate cortex, that tell you that that is really bad.
It gives you the unpleasant feeling, the emotional component of pain.
And you know, they’re interesting.
There are patients who dissociate between the two.
So there are patients who, let’s say, have a stroke or a tumor in the insula or in the cingulate cortex.
And you’ll have these patients and they’ll tell you, you know, I know you’re hurting like my finger and I can tell you that it is my finger, but it doesn’t feel unpleasant at all.
So these pathways are dissociated in the brain.
Now in the work that we’ve done, we’ve reconstructed the basic pathway that just processes pain stimuli, not the emotional component.
So we wouldn’t say that they’re feeling pain in any way, right, just to make it clear.
Because as you can imagine, there are all kinds of other ethical issues that are arising from like most of the work that we do, obviously, because, you know, we want our models to be closer to the human brain because we think that many of the psychiatric disorders are uniquely human.
And yet the more the closer they are to the human brain, the more uncomfortable we feel, right?
So I think it’s so like mitigating this risk moving forward that I think is very important.
How do you now, having had this experience with the sensory aspect of it, reverse engineer again the way to get a drug to alleviate the hypersensitivity to pain?
Sure.
I mean, there are many ways that you can do this.
So like, now think about it.
It’s called scotch.
Well, no, everybody’s got a thing with opioids.
But there must be a mechanism there where opioids use that you can sort of tag onto, but not get that addictive part.
Exactly.
And think about it.
Like, it’s sad that the best treatment that we have for like pain comes out of like poppy seeds.
And what discovered thousands of years ago by chance, right?
I mean, essentially piggybacks on the circuit does not come from a deep understanding of the circuit itself.
Of the circuit.
Don’t you make opium from poppy seeds?
Yes.
Okay, I just want to clarify that.
So I think the idea now is that we have the circuit in a dish.
You can add opioids, by the way, and see how they modulate this, and see, okay, this is what opioids do to the circuit.
But let’s now try to do the same thing in a different way.
One that is sort of like, you know, driven by the biology behind it.
And I think that’s the beauty of it.
That is the beauty.
And by the way, professor, if you ever get to that place, please email me right before you make that public, because I would like to be the first investor in the pain-free opiate that is non-addictive, because that is, I mean, that’s the end of the game right there.
And just to be clear, Chuck.
What?
Because when my hand was, I just wanted to, like, get back to my hand in the ice bucket.
In the bucket, yeah.
Okay, long ago, when I began wrestling in high school.
And I was going to bring this up.
I think it’s because you were an athlete, and athletes have to deal with pain all the time.
Exactly, and I judge, by looking at the situation, is this pain something that will cause irreparable damage?
Or is it just simply pain?
Okay, and I’m looking at it, my hand is in a bucket of ice.
Yeah, it hurts, but who cares?
I’m not gonna get frostbite from it, okay?
So.
See, you and Gary have that, I’m sure, because Gary’s had a ton of surgeries.
Oh yeah, we’ve had ice buckets.
He’s played in pain, he sat in ice after games, right?
See, and I have not played any real.
I’ve done none of that.
I’ve done none of that.
And this is, you put-
That’s why you wimped out in the time of need.
Because this is how pain works for me, okay?
The way pain works for me is I experience it, and then my brain, my body, and everything in my soul goes, Jesus, no!
Please, Lord, no!
So.
Oh, that’s what-
Okay.
So, when you’re saying you’re building these avatars, and the detective work that comes, are you finding more clues and more answers, or are you just finding clues and then we got to sit there, scratch our heads, and hopefully come up with an answer?
Or is this really empowering the sort of psychiatric research that you’re interested in?
You know, the way I look at it is, you know, psychiatric disorders have been a mystery, like, no doubt.
I mean, how does complex behavior or hallucination arises from the brain in mesmerizing us for such a long time?
And it’s almost like, if you were to think about it, it’s almost as like seeing, you know, Egyptians writing for the first time, right?
You look at them, you know, where do you even start?
I mean, they’re beautiful drawings, you know, you could classify them based on, like, the animals, but then you can’t make sense of what the meaning is.
And you see, that’s why, you know, if you think about it, like, historically, the discovery of the Rosetta Stone, right?
Like, this tiny piece tablet that, for the first time, had hieroglyphs on one side and Greek writing on the other one, right?
And then, you know, this French scientist, who came with Napoleon, finds this, starts looking at it, and that becomes essentially the, you know, enabling tool.
Suddenly, we could actually see what word does what.
And you know, the cool thing about the Rosetta Stone?
It’s like a shopping list or something.
It’s like, it’s not any deep.
Just like brown eggs.
I don’t know if it’s exactly a shopping list, but it’s something completely mundane.
Something very, very, yeah.
Something really trivial, absolutely.
And yet, like, it was the only writing that we know that had both on both sides.
So, I think the question is, we need to somehow translate at one point.
So, like, these mental processes that are so complex into what we can deal with, which is really molecular biology.
That’s what we can control.
Molecular biology we can control.
So, I think, to a large extent, I’ve seen this, like, the mission for my lab and in general, like, I think, for the community more broadly, is really to try to translate some of this complex phenomena of the brain in very simple processes, calcium and a neuron, two neurons connecting with each other.
And then, hopefully, by doing that and finding ways of reversing it, those will also reverse or at least improve.
We don’t know that.
You know, that has not yet been done.
And we’ll have to see whether a clinical trial will actually be successful.
I mean, we’re preparing for a clinical trial for Timmons syndrome right now.
We’re still, like, in the last stages of preparation.
We found most of the patients in the world were building a special unit here at Stanford where we’re going to be hopefully bringing them in the next year or so and doing a clinical trial.
So we’ll see.
And then, you know, this is the first disease.
I mean, and I look at Timmons syndrome as like really being the first first.
But we have half a dozen of other conditions that we’ve been studying from various angles.
Really, I mean, I see this this is going to be the golden age for human neuroscience.
And I’m delighted to learn that you’re putting in this much effort for a disease that is so rare.
Yeah.
I mean, think about that.
So the rarity, at least the people have the benefit of your attention given to it.
Yeah.
Rather than someone just making the cost-benefit analysis.
And saying we’re not we’re not doing anything.
We’re not going there.
We’re not going down that road.
So are we saying here that your Assembloid research and work is going to be the key to understanding what has been hidden brain biology?
How soon do you think maybe you really will be able to not just tick off the Timothy Syndrome but take on other horrific diseases?
Oh, we’re already working on others.
I mean, you know, at least half a dozen we’ve been studying.
Like some are associated with epilepsy, some with intellectual disability.
We have a few forms of schizophrenia.
So we’ve been deploying this like systematically.
And another thing that we’ve done, to be honest, and this is sort of like being in the spirit of what we do at Stanford, is, you know, I lead a center here.
And in the beginning, it was, you know, when we published some of the first methods, everybody was like, oh, you know, can we come to the lab and learn how to do it?
Like we want to do it too.
And we brought people here initially, but then at one point, you know, we couldn’t train enough people.
So we actually started doing literally courses where we bring students from all over the world from various labs and for about a week, almost like in a cooking show, if you want to think about it, right?
Because, you know, the experiments are done before.
We just show them, these are the critical steps that you need to do.
And so we’ve been helping more than 300 labs around the world to, you know, implement this method.
And if the breakthrough is not going to come from my lab, therapeutically speaking, that’s fine, because it will probably come from somebody else, somewhere like, you know, in a corner of Europe or who knows of South America, doing experiments on a rare form of disease and finding a therapeutic, that would be fine, I think.
Because there’s so much to do for, you know, one in four individuals suffers from a psychiatric disease today.
It’s a huge burden.
Are we going to come across a situation where you are going to be faced with building an assembloid or creating an assembloid that will just be too complex?
Is there a limit to what you can assemble?
There are absolutely limits to what we can assemble.
And, you know, while like many of the features of this assembloids are really fascinating and surprising, you know, they still have a lot of limitations.
You know, I mean, they’re not vascularized.
They don’t receive blood supply.
We may be able to stimulate them with like capsaicin or something else, but they’re not receiving the rich sensory information that is important.
You know, think about the, you know, if you have a kitten where you, you know, you cover one eye, you cover that eye for a week, that cat will never see with that eye.
You do it in an adult cat for a week, no problem whatsoever.
So early in development, if some of the circuits do not receive the right input, they won’t develop properly.
So, you know, again, while our models are relatively complex already, they lack a lot of the complexity.
And, you know, as George Box famously said, that all models are wrong or some are useful, you know, and the models that we make are not…
Our goal is not to make a perfect model of the brain.
It’s like to make a good enough model of a part of a brain or of a circuit that will give us the breakthrough therapeutically.
I wouldn’t be so harsh with the term model there.
I would say all models are almost by construct incomplete.
Right.
But that wouldn’t make them wrong, necessarily.
They’re just…
They’re not the whole story.
That’s why they’re a model.
That’s why they’re a model.
Because otherwise it would be the exact thing.
You wouldn’t need it.
You wouldn’t need a model if you could replicate the exact thing.
It would be the thing itself.
Right.
I just love that Assembloids sounds like a Cartoon Network show.
Like Assembloids, weekdays at 3, right after Transformers.
Actually, that’s how there is a game.
There is a video game for it, which I didn’t know when I put out the term.
But there is a very popular video game that is literally called Assembloids.
Cool.
Where do we hit the ethical wall and hit the regulatory and all the other things?
Did you say regulatory?
I did.
This is America, Jack.
It’s regulatory.
Regulatory.
Not even regula…
Regulatory.
I didn’t come here for a lecture on geography.
I know it’s America.
It’s America, Jack.
Anyway, he was saying…
You know, we think about this like all the time.
Honestly, in the beginning, obviously, there are like not that many ethical issues.
But as we’ve progressed, it becomes clear that we have to think carefully.
So they’re like, you know, the way I think of it is like in multiple levels.
Like on one hand, there are like issues about the cells.
These are human cells that we’re using.
Who owns the cells?
You have to give consent for this experiment to be done.
And we do that all the time.
And so we always have to put that into the context of like, what are we doing with the cells?
What the cells were consented for?
That’s very much like, who is the woman who…
Wax.
What’s her name?
Wax.
Wax, yes, whose cancer cells were used for decades and saved and created many breakthroughs in cancer and the family got nothing and she never gave permission.
So it’s good to know you’re doing that.
And that’s why it’s critical every time we collect the skin.
Henrietta Wax, yes.
You know, the patients or the parents in the case if they’re minors will actually be clearly informed about what will happen with the cells, how the cells will be shared with others, for instance, under what conditions and so on and so forth.
So on one hand, there are like this issues about the cells.
Then sometimes, as you know, we’re using animals.
So sometimes we transplant this into the animal.
So we also have to think about the welfare of an animal.
I mean, you transplant more, is the animal suffering in any way?
And then the third problem, which is perhaps the more philosophical in a way, is like, are there any new emergent properties?
Like, are there, you know, features, complex features that are rising from this that would make one thing that we need to regulate this field?
Currently, I think the models that we have in VITRO are not sufficiently complex to justify, you know, the presence of any complex features.
Like, that’s why we don’t use the term, generally, you know, the term intelligence for this, because intelligence is really a property of an organism.
It involves, like, goal-directed behavior and involves learning.
None of our cultures do that.
And using, you know, anthropomorphizing, it’s not generally a very useful thing to do in this case.
But as the models become more complex, we have to start having these conversations.
And that’s why, you know, last year, we had an Aselomar meeting, which is like this historic place here in California, you may have heard, where many of these ethical discussions have started in biology.
In the 70s, when cloning, gene cloning was so, like, discovered, then everybody was like, what is going to happen?
We’re now modifying these genes, and we’re going to create new organisms.
So scientists got together there with philosophers, you know, with journalists, so that’s what we’re also doing now.
We’re getting together a larger group and thinking, what are some of the implications, you know, sociologically, religiously, philosophically, while at the same time thinking that psychiatric disorders are a huge burden.
And if you have a technology that has the potential to change that, to provide cures, is it, you know, unethical not to use it?
Right, I mean, there’s even that argument, you know, where, you know, how far do we go in that?
So those are like ongoing discussions.
I mean, it’s been really interesting.
I spend more and more of my time as part of these conversations.
Let me take just one other place before we land the plane here.
You came into this as an expert in the autism spectrum patients.
And a new term that’s been bandied about for the last certainly 10 years is the concept of neurodiversity.
When you look at it that way, who is anyone to say that someone needs repair if they’re simply manifesting on a spectrum of neurodiversity?
Your counterparts, not long ago, would have labeled homosexuality as a mental disorder in need of repair.
And only recently, in historical times recently, was that removed from the list of human maladies and disorders.
So there’s an ethical, another ethical frontier about what it is you judge needs repair versus is just another kind of person.
And that’s absolutely one of the discussions that we’ve been having, one of the ethical discussions that we’ve been having.
And you know, all psychiatric disorders are on a spectrum with the population.
And some of them are more severe and some of them are less severe.
And that’s also the case for autism.
You know, autism is certainly a spectrum.
But what we’re focusing on is actually what we now call profound autism.
This is the autism that is really debilitating.
So patients with Timmith syndrome or like some of the other patients that were like with other disorders, can have 60 seizures a day.
Oh my, really?
They are unable to make any eye contact.
They need a caregiver for the rest of their life.
You know, the biggest fear that a parent has when they have a child is like, what if I die?
So I am seeing it through the eyes of some of these parents.
They’re dealing with like really the devastating forms of autism, what we call profound autism.
And then of course, there is like what you mentioned, which are neurotypical or, you know, aspects of how we interact with each other that do not require any, nobody wants to cure or to provide treatments for anybody.
But these patients are severely affected.
Most patients with psychiatric disorders are severely affected.
Because I once asked Oliver Sacks, who is a friend of our show, we have some archival content with him.
Oh, that’s amazing.
Yeah, yeah.
I asked him after a public talk that he gave, if you could go back in time and carry with you a pill that would cure your own ailments.
He had sort of certain neuro issues.
He has, correct me on the word here, prognoplagnesia.
Yes, he did, yeah, he couldn’t really identify.
He had face blindness, okay?
Oh, wow.
And some other elements to it.
I sometimes wish I had that.
Okay, so I asked him, if you could take a pill that would just cure that back when you were 17, would you, looking back at that time?
And he said no, because it was that, those differences in the way his mind worked that got him interested in neuroscience in the first place.
That was his destiny.
But you see, that’s exactly the point where we started.
The beauty of building a nervous system is that while there is a basic plan that makes our brains the same, we pretty much can do the same things, it also creates a lot of diversity.
Even monozygotic twins have the same genetic material, they share the same womb.
And then they can have different sexual orientations.
They like, they have different hobbies.
They have different fingerprints, if I remember correctly, don’t they?
They do, they do, yes.
Because again, there is a lot of stochastic forces in development.
And those are the ones that make us different.
And that’s how evolution actually works too.
You know, by selecting these differences, that to a large extent probably that’s what made us as a species so successful.
The fact that there is always an individual who has a vision, who wants to go and discover a new continent.
So I think that’s the power of our species.
And I think I know very few, honestly, psychiatrists or neurologists who would want to cure that or change that.
I think what we’re dealing really on the field is really these devastated conditions that make essentially most of these children unable to really function as adults or as children.
So this is a very human-centric view.
So if you were the COVID virus, you would say, let’s invent humans who then have airplanes so that we can cross continents and affect other people.
Absolutely.
They are the true owners of this planet.
Let’s be honest, viruses, they’re true owners of this planet.
You said it, microbes, we’re just an Uber.
Yeah, that’s all.
We’re just an Uber ride.
Uber ride.
Well, Sergiu, it’s been a delight to have you on StarTalk, sharing your expertise with us and taking time out of what we know is your busy research schedule.
Give us a little glimpse into what you’re doing in your lab.
Just congratulations to you and all the people who work in your lab, who are surely working there right now while you’re talking to us.
They are, right here, I mean, yeah.
Yeah, and really, they’re the ones that are doing all the work.
I mean, this work, hopefully it came through from the discussions, but these experiments are long.
I mean, they last hundreds of days.
Because human development takes a long time.
So, it requires a lot of dedication.
But I think the promise of what this could yield, ultimately, you know, understanding the human brain, is, you know, is addictive.
So, you know, you really want to figure this out.
Well, thank you again.
I’d like to reflect on this with a brief cosmic perspective, if I may.
Please.
This moving neuroscience frontier has got me thinking.
You look at the progress of civilization.
It always comes about when we have the proper match between a tool and a goal.
And when they come together, we build things that didn’t exist before.
Or we disassemble things that had never been taken apart before.
But in all cases, it has to do with the precision of the tools you bring to the task.
And to learn what’s going on on the frontier of neuroscience, it feels to me that it’s finally catching up with the methods and tools that have shaped engineering throughout the history of civilization.
Engineers built dams and buildings and aqueducts and everything that we value and care about in our modern lives.
But the time has come to care about what’s going on inside our brains, within our minds, and I’m delighted to learn that that is a frontier that finally has tools befitting the task.
Welcome to the club, neuroscience.
And that is a Cosmic Perspective.

 Unlock with Patreon
Unlock with Patreon



 Become a Patron
Become a Patron